Call for Input – pembrolizumab (KEYTRUDA®) for ovarian, fallopian tube, and peritoneal cancer
Have you or your loved one been treated with pembrolizumab (KEYTRUDA®) for managing ovarian, fallopian tube, or primary peritoneal cancer? We need your help! Pembrolizumab, in combination with paclitaxel (with or without bevacizumab) is undergoing a federal funding review for the treatment of adults with platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancers whose tumours are PD-L1 positive and who have received one or two prior systemic treatment regimens. The Canadian Cancer Survivor Network invites you to share your experience in a 15-minute anonymous survey to inform our patient evidence submission to Canada’s Drug Agency. The survey will remain open until Thursday March 12, 2026. Learn more and take the survey here! <Call to Action button text with hyperlink to https://www.surveymonkey.com/r/W26ovarian or replace the ! with : and include the link instead> We welcome and encourage participation from non-Canadians and those who do not strictly match every criterion, too. Your responses will help us build a complete and meaningful submission. Thank you for your participation! Your insights can help improve access.
Sociodemographic and Psychosocial Patient and Physician Factors in Oncology Treatment Decision-Making
Hello, Researchers at the University of Calgary are conducting a study to understand how patient and physician factors – like their background, experiences, and personal beliefs – affect the way they make treatment decisions together. We also want to understand how these decisions impact patient’s health. We are currently seeking research participants for this study. Participation in this study is completely voluntary and you are free to change your mind at any time. Who is eligible? If you are over 18 years old, living in Alberta, have received a cancer diagnosis within the past two years, and been referred to a medical oncologist for a chemotherapy assessment, you may be eligible for this research study. What will you be asked to do? You will be asked to:
- Complete 2 questionnaires about your socio-demographic and psychosocial factors, as well as a few questions related to your cancer diagnosis
- Attend and actively participate in one focus group (approximately 90 minutes)
UBC Research Study Men’s Health Prostate Cancer Survey
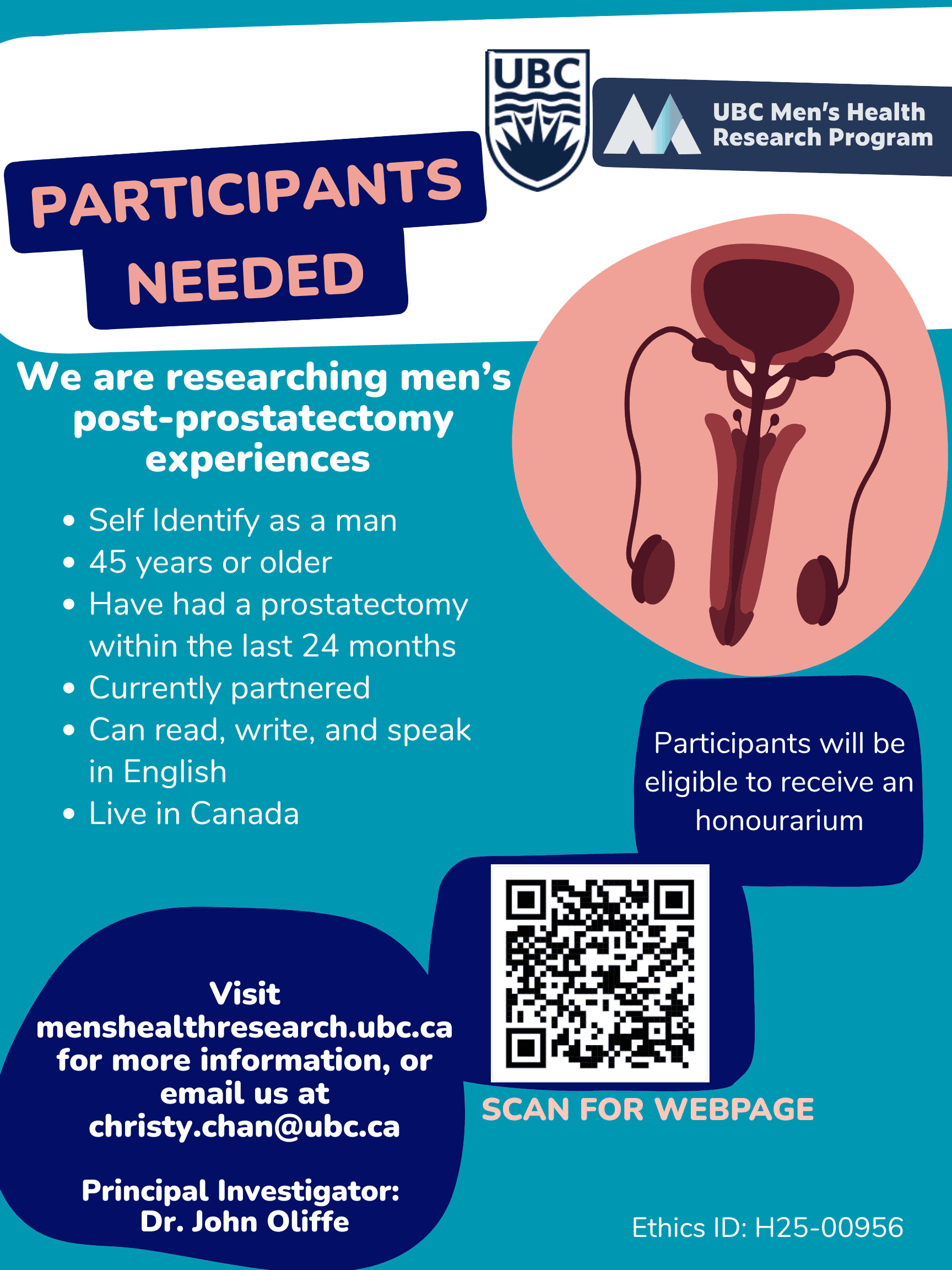 Looking to share your experience and contribute to research? The UBC Men's Health Research Program is seeking men 45+ who have undergone a prostatectomy within the past 24 months to participate in a study exploring post-prostatectomy experiences. If you're currently partnered, can communicate in English, and live in Canada, you may be eligible to participate and receive an honorarium for your time. Your insights could help improve care and support other men navigating this journey. Visit menshealthresearch.ubc.ca or contact christy.chan@ubc.ca to learn more.
Looking to share your experience and contribute to research? The UBC Men's Health Research Program is seeking men 45+ who have undergone a prostatectomy within the past 24 months to participate in a study exploring post-prostatectomy experiences. If you're currently partnered, can communicate in English, and live in Canada, you may be eligible to participate and receive an honorarium for your time. Your insights could help improve care and support other men navigating this journey. Visit menshealthresearch.ubc.ca or contact christy.chan@ubc.ca to learn more.Survey for Patients with Follicular Lymphoma
From Lymphoma Canada
 If you are a patient with relapsed/refractory follicular lymphoma and have been treated with Mosunetuzumab (Lunsumio) or Tafasitamab (Minjuvi), you can help by completing our survey.
Lymphoma Canada is preparing 2 submissions for Canada’s Drug Agency (CDA). This survey provides us with the patient input required for the submission. CDA uses this information to help them make recommendations to the provinces and territories regarding funding for new cancer drugs.
The therapies that will soon be reviewed by the CDA are:
If you are a patient with relapsed/refractory follicular lymphoma and have been treated with Mosunetuzumab (Lunsumio) or Tafasitamab (Minjuvi), you can help by completing our survey.
Lymphoma Canada is preparing 2 submissions for Canada’s Drug Agency (CDA). This survey provides us with the patient input required for the submission. CDA uses this information to help them make recommendations to the provinces and territories regarding funding for new cancer drugs.
The therapies that will soon be reviewed by the CDA are:
- Mosunetuzumab (Lunsumio) for the treatment of adult patients with relapsed or refractory follicular lymphoma who have received at least two prior systemic therapies.
- Tafasitamab (Minjuvi) in combination with rituximab and lenalidomide for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL).
CANADA WIDE ACCESS TO CARE SURVEY
From the Canadian Neuroendocrine Tumour Society. You are receiving this voluntary survey because you are registered with the Canadian Neuroendocrine Tumor Society (CNETS) and have agreed to be contacted. CNETS is reaching out to its community of patients, caregivers, and advocates to better understand the current challenges in diagnosing and accessing care for neuroendocrine cancer (NET) across Canada. We know that access to timely and appropriate testing, diagnosis, treatment, and ongoing care is not always straightforward—and we want to hear directly from you. The Canadian healthcare system is under growing pressure, and this can impact how quickly people are diagnosed and how consistently they receive follow-up care. By gathering firsthand experiences, both positive and challenging, we aim to identify common barriers, uncover best practices, and explore ways CNETS can advocate for improvements in the patient journey. This anonymous survey contains up to 48 questions and will take approximately 20 minutes to complete. It is available in English and French. The questions will cover aspects of your care experience, access to diagnostic services, timelines, and support, among other topics. You may complete the survey independently or with help from a trusted family member, friend, or caregiver. Who can complete this survey? Any Canadian living with neuroendocrine cancer who has received or is receiving care in Canada is welcome to participate. A caregiver or representative may also complete the survey on behalf of a NET patient. If doing so, please ensure that all responses reflect the patient’s experience and not your own demographic information. Participation in this survey is voluntary and completely confidential. You may exit the survey at any time and your answers will be deleted. Your responses will remain anonymous and will not impact the care you receive. At the end of the survey, you will have the option to either submit or void your answers. Only completed surveys will be analyzed. By completing the survey and choosing to submit your answers, you are consenting to the use of this anonymous information in our study. Neither CNETS nor the study team will have access to any identifying information. This survey is the only component of this project. The only other time you may be contacted is if you opt to receive the published results when they become available. For any questions or concerns, please contact: miranda.stavrides@cnets.ca with the subject line RE: Access to Care Survey. Thank you for your time and your willingness to share your experience. Together, we can make a difference in the future of NET care in Canada.
Survey for Patients with Hodgkin Lymphoma
From Lymphoma Canada If you were newly diagnosed with Hodgkin Lymphoma and have been treated with Brentuximab vedotin containing regimen (BrECADD), you can help by completing our survey. Lymphoma Canada is preparing a submission for Canada’s Drug Agency (CDA). This survey provides us with the patient input required for the submission. CDA uses this information to help them make recommendations to the provinces and territories regarding funding for new cancer drugs. The therapy that will soon be reviewed by the CDA is the Brentuximab vedotin containing regimen (BrECADD), which includes etoposide, cyclophosphamide, doxorubicin, dacarbazine, and dexamethasone, for newly diagnosed Hodgkin Lymphoma patients. You do not need to live in Canada to complete this survey. You can still participate in this survey if you are a Hodgkin Lymphoma patient who has not received this therapy. BY COMPLETING THIS SURVEY, YOU ARE PART OF THE PROCESS THAT MAY HELP PATIENTS GAIN ACCESS TO THIS TREATMENT IN CANADA. It should only take between 15-20 minutes of your time. Please share and help us spread the word. Thank you for your support.
Lakehead University Research Study
Survey on Travel for Treatment
 Did you or a loved one (adult) need to leave your home town or city for cancer treatment in the past 5 years? If so, were your finances, employment, housing, education, family life, or social support impacted? Your voice is needed!
We are sharing an important survey from one of our partners in cancer advocacy, the Leukemia & Lymphoma Society of Canada (LLSC), who have circulated this survey already to individuals impacted by a blood cancer.
The survey, “Leaving Your Community for Cancer Treatment: Understanding the Impact,” is now open to Canadians, 18 years or older, who have been impacted by any type of cancer, other than blood cancer - and their caregivers.
Results, from feedback like yours, will increase awareness of the impacts of cancer treatment access, and generate discussion across Canada, including with authorities who make decisions about public funding for cancer drugs.
We urge you to consider taking this survey; your participation will provide much needed attention to access to cancer treatment in Canada. Thank you in advance
Did you or a loved one (adult) need to leave your home town or city for cancer treatment in the past 5 years? If so, were your finances, employment, housing, education, family life, or social support impacted? Your voice is needed!
We are sharing an important survey from one of our partners in cancer advocacy, the Leukemia & Lymphoma Society of Canada (LLSC), who have circulated this survey already to individuals impacted by a blood cancer.
The survey, “Leaving Your Community for Cancer Treatment: Understanding the Impact,” is now open to Canadians, 18 years or older, who have been impacted by any type of cancer, other than blood cancer - and their caregivers.
Results, from feedback like yours, will increase awareness of the impacts of cancer treatment access, and generate discussion across Canada, including with authorities who make decisions about public funding for cancer drugs.
We urge you to consider taking this survey; your participation will provide much needed attention to access to cancer treatment in Canada. Thank you in advance University of Alberta REACH Study

Sedentary Behaviour Study for Patients

Sedentary Behaviour Study for Caregivers

Nova Scotia Health Breast Cancer Survey
Researchers at Nova Scotia Health want to hear from female breast cancer survivors who are experiencing issues with sexual function after completing their cancer treatment. The researchers want to explore whether and how women seek help for these issues and investigate the impact these issues have on their lives. Issues with sexual function are common in breast cancer survivors after their initial curative treatment. These issues may be characterized by vaginal dryness, painful intercourse, hot flashes, decreased sexual interest, decreased arousal, difficulty attaining orgasm, and poor body image. Though these issues are common after breast cancer treatment, many women face barriers when attempting to seek care and support. In order to improve access to care, we need to have a better understanding of the current help-seeking strategies used by breast cancer survivors, as well as how issues with sexual function impact their daily lives. The researchers hope the findings from this study will inform future research on the development of interventions that improve holistic care for female breast cancer survivors after cancer treatment. You are eligible to participate in this study if you:
- are at least 18 years of age;
- reside in Canada
- are female
- have been treated for breast cancer in the past 10 years.
Improving communication about low risk ductal carcinoma in situ (DCIS)
Dr. Anna R Gagliardi, Senior Scientist at the University Health Network, is conducting a Canadian Cancer Society funded study to identify non-cancer labels for Ductal carcinoma in situ (DCIS), and is inviting you along with other women from across Canada who had DCIS, to shape recommendations on the labels, language, and other strategies that physicians should use to improve communication about DCIS. This may reduce confusion and anxiety among women with low-risk forms of DCIS. This study involves completing a two-part survey that, in total, will require about 20 minutes of your time over the next month. First, read the two documents attached: a one-page summary of our research and a list of the survey items. Then, if you are interested in participating or wish to learn more about the study, please contact the study coordinator Mavis.Lyons@uhn.ca. Participation in this study is completely voluntary. Infographic Information Sheet
Nuance: Inequity in Aging & Cancer
This study is put on by the Princess Margaret Cancer Foundation The overall objective of this study is: - To gain a better understanding of the factors impacting access to cancer care and clinical research for racialized older adults - To identify strategies to address disparities in these areas The Why Cancer predominantly affects older adults - US data show that there are disparities based on age, race, and sex/gender with regards to treatment outcomes and clinical trials participation - Black persons particularly, and to a lesser extent Asians and Hispanics, do worse than Whites - There is No systematic collection of data on race or gender in Canada - “Until we measure it and understand it, we cannot fix it” Who are we looking for to participate? Older adults (60+ years) who identify as Black, South Asian (e.g. India, Pakistan, Bangladesh), OR East Asian (e.g. China, Japan, Korea, Vietnam) AND }Have had a cancer diagnosis in the last 10 years What do I do if I want to participate? - Let us know! (see below for contact info) - Review and sign consent form - Fill out an online OR paper survey asking about your experience accessing cancer care and clinical research - About 15-20 minutes to complete - $10 gift card to thank you for your participation - All responses will be treated confidentially - Optional interview in a future phase - If you are interested in participating, please email Sara Durbano to receive links to the study consent form and survey (online or paper) sara.durbano@uhn.ca Regards Shabbir Alibhai
Broadstreet Cancer Treatment Survey
Dear member: We are conducting a new research study in which you may be interested in participating. We are aiming to understand the thoughts and opinions of Canadians on how new cancer treatments are evaluated, and how decisions about the availability of new treatments should be made. We will be conducting virtual interviews with 20-25 interested and eligible individuals. Virtual interviews will occur in March and April 2024, and participants will be compensated $100 for their time and expertise. To participate you need to:
- Be 18 years of age or older
- Be currently undergoing or having had past treatment for stage I-III breast cancer; stage I-III non-small cell lung cancer; limited stage small cell lung cancer; or stage A or B liver cancer
- Live in Canada
- Be fluent in English
- Complete an eligibility questionnaire about cancer diagnosis and treatment
Interview Opportunity For Adults With Metastatic Urothelial Carcinoma (Bladder) Cancer
Click here for full details CONTACT: dlambkin@marketresearch2go.com
Seeking patients who had low-risk cancer for a research study requiring one telephone call
This study is commissioned with the University Health Network in partnership with the Canadian Cancer Society Background: Due to more screening, many more people are diagnosed with abnormal cells with a very low risk of ever turning into cancer. Many patients undergoing treatment for these abnormal cells believe they have cancer, which causes anxiety and reduces quality of life. Little prior research has explored how to improve communication about “low-risk” cancers so that patients better understand their diagnosis. Study purpose: Identify ideal ways to name and discuss low-risk cancers by asking patients and doctors about the names they prefer for low-risk cancers of the breast, cervix, bladder, thyroid, and prostate, and the reason for those preferences. You can participate if you: ? Are 30 years of age or older ? Were diagnosed in the last 5 years with abnormal cells in any of the following body parts that required only regular monitoring because of a very low chance of ever turning into cancer, or if treated, would not likely recur: o Breast (only ductal carcinoma in situ) o Cervix o Bladder o Thyroid o Prostate ? Live anywhere in Canada ? Can understand and speak English language What you will be asked to do: ? Read and sign an online consent form ? Participate in a single telephone interview of about 20 minutes ? We will ask for your opinion about the words used to name and describe abnormal cells in the breast, cervix, bladder, prostate, or thyroid What we will give you: ? After you participate in the telephone interview, we will send you compensation ? When the study is done, we will send you a summary of the results To express interest or ask questions: The research study is being conducted by Dr. Anna Gagliardi from the University Health Network (UHN) in Toronto with funding from the Canadian Cancer Society. If you are interested in participating or wish to learn more about the study, please contact the study coordinator: Mavis. Lyons@uhn.ca. Please note that information sent by email may be accessible by external parties and therefore not entirely secure. Please do not communicate personal sensitive information via e-mail.
SEAMLESS Study: A Smartphone App-based Mindfulness Intervention for Cancer Survivors
This study is commissioned by the University of Calgary and Alberta Health Services Click below for more information: SEAMLESS Flyer_Apr2023 SEAMLESS Email Flyer
Lung Cancer Screening Values (LCSV) study team at McMaster University
We are recruiting 55 and 85-year-olds who can converse in English for a research study. The purpose of this study is to investigate Ontarians’ values, attitudes, and beliefs about lung cancer screening. Participants will be compensated $20 for completing an interview in person (Hamilton, ON), by telephone or video call. If you are interested in participating, please fill out the eligibility survey here: https://dfmgp.mcmaster.ca/surveys/?s=MXFWX8483DJ88RLW We hope to learn what you think about the importance of lung cancer screening, who you think should be screened, and your perspectives on the benefits and harms of lung cancer screening. Your participation can help advance our thinking about lung cancer screening in order to make a potential future lung cancer screening program in Ontario fair, effective, and an efficient use of health care dollars. This research study is part of a doctoral thesis by Manisha Pahwa, a Ph.D. student at McMaster University, and has been reviewed by the Hamilton Integrated Research Ethics Board (project #8310)”
Bladder Cancer Patient Feedback
Bladder Cancer Canada is looking for patients to provide input for 4 different studies, including two studies directly conducted by doctors.These studies are looking to gain insights from Canadians diagnosed with advanced (metastatic) bladder cancer and muscle-invasive bladder cancer to better understand their personal experiences from the time of diagnosis and beyond.Selected participants will be invited to participate in a virtual meeting or complete a survey to share their perspectives with other attendees and/or researchers. Compensation is provided for selected studies.We would truly appreciate it if you could inform any bladder cancer patients of these studies.Anyone interested can connect with Michelle Colero, who can provide more information - michellec@bladdercancercanada.org. Evaluating Psychosocial Needs of Retinoblastoma Patients and Families
Dr. Helen Dimaras (Scientist and Director of Global Eye Health Research, The Hospital for Sick Children, Toronto) is?partnering with researchers, clinicians, and retinoblastoma patients and families to develop a study that will uncover the unmet psychosocial needs of Canadian retinoblastoma patients and families.
Fear of Cancer Recurrence
This is a doctoral research project done by Jani Lamarche, PHD candidate in clinical psychology at the University of Ottawa. Lamarche is looking to adapt and provide a group intervention aimed at reducing fear of cancer recurrence in family caregivers of cancer survivors. To participate, email jlama023@uottawa.ca Fear of Cancer Recurrence_Recruitment Flyer
University Of Liverpool Research Participants Call Out
The University Of Liverpool is seeking participants for research into how young women of color experience hair loss as a result of chemotherapy for breast cancer. Are you a woman of color between the ages of 21 and 45 who experienced hair loss as a result of chemotherapy for breast cancer (more…)
University of Calgary Exercise for Cancer to Enhance Living Well Health and Wellness Lab
Exercise for Cancer to Enhance Living Well (EXCEL) is a five-year nationwide project to increase the accessibility of exercise programs for rural and remote cancer survivors, (more…)
McGill University LymFit Exercise study.
Researchers at McGill University (Montreal, QC) are leading an exercise intervention study for young adults with lymphoma to get them to engaged in physical activity and increase their overall fitness and quality of life. (more…)
Bladder Cancer Canada Bladder Cancer Patient Insights Survey
Bladder Cancer Canada is looking for bladder cancer patients and caregivers to complete their to ensure they are supporting patients in the best way possible. (more…)
EXCEL Program: Exercise for Cancer to Enhance Living Well
The EXCEL program is open to all adults affected by cancer who do not have access to exercise oncology programs in their area (which is most as there are very few established exercise oncology programs in Canada). We'd love for more Young Adults to participate in the program. Programs start every 12 weeks in Fall (sept start), Winter (Jan start), Spring (Apr start), and summer (July start, shorter program). For more info, visit this link: https://kinesiology.ucalgary.ca/labs/health-and-wellness/research-projects/excercise-cancer-enhance-living-well-excel EXCEL_Poster_ParticipantAction_202110 ~
RECRUITMENT FOR A RESEARCH STUDY: Managing Your Caregiver Worries
Do you worry about your loved one’s cancer coming back? Do you worry weeks before your loved one’s follow-up appointments? Do your worries about your loved one’s cancer coming back cause you distress or affect your daily life? If so, a brief online group study is being offered to address these worries. (more…)
Symptom Tracking & Management to Improve Cancer Care
e-IMPAQc is inviting primary support persons (caregivers) to individuals diagnosed with cancer to an online focus group or one-on-one interview for a research study and would like to hear from a large and diverse group.
We invite you to share your needs and challenges as the primary support person to a family member or friend diagnosed with cancer by participating in two 90-minute online focus groups and one 120-minute online focus group with the research team of Sylvie Lambert of McGill University. Your feedback and suggestions will help the e-IMPAQc team develop a mobile application to provide cancer caregivers with educational materials and resources specific to your needs. Participants receive $35 pr workshop as a thank you for their contribution. For more information contact the team at e-impaqc_postt@mcgill.ca and visit https://e-impaqc.com/en/~Call for Stories about Dealing with Side Effects of Prostate Cancer Treatment
Urinary incontinence and erectile dysfunction are two very common side effects of treatments for prostate cancer. They frequently persist for months or years after treatment. These side effects can seriously worsen patients’ quality of life. CCSN is undertaking a project to raise awareness of the need for rehabilitation services for prostate cancer survivors who experience these life-changing side effects, and to help make sure patients know about rehabilitation options that already exist. As part of this project, we are looking for stories from prostate cancer patients and survivors about dealing with incontinence or ED after treatment. These stories will be published in our updated prostate cancer section on our website. If you want to contribute a story, send us an email at info@survivornet.ca.
Call for Stories of Experience with COVID-19 and Cancer
CCSN's surveys on the impact of the COVID-19 pandemic on access to cancer care have given us a lot of information about the numerous ways in which the pandemic has caused difficulties for cancer patients, caregivers, and pre-diagnosis patients. However, we're also interested in learning more specifically about the experience of cancer patients who were ill with COVID – fears and concerns, interactions with the healthcare system, and any other challenges or difficulties. If you are someone who has had cancer and you got COVID, and want to share your story, send us an email at info@survivornet.ca.
Lung Cancer Support Survey
The Lung Health Foundation is committed to delivering a support service to Lung Cancer patients and caregivers that is current, relevant and meaningful. With that goal in mind, they have created a survey to gather input and insights from those with lived experience to increase our understanding of the lung cancer journey, including diagnosis, navigation of care and gaps in support. This survey will take a few minutes to complete. ~





